VIRC Translational Approach
VIRC Translational Approach
The Director of the National Institutes of Health recently stated that “The average length of time from target discovery to approval of a new drug [for human use] currently averages about 13 years, the failure rate exceeds 95 percent, and the cost per successful drug exceeds $1 billion, after adjusting for all of the failures“. This profound statement points to a general concern about the current state of drug development. There are several reasons why clinical trials may fail and they include:
- Poor translation from an “induced” animal model to humans. In an induced model, animals are purposely injured and then treated with a potential therapeutic to determine its efficacy. These models do not always recapitulate the pathogenesis observed in humans and are limited in their genetic and/or physiologic diversity.
- Failure to develop an adequate and validated process to scale up drug production without changing the potency/efficacy of the drug.
- Poor understanding of the mechanism of action of the drug in treating a particular disease.
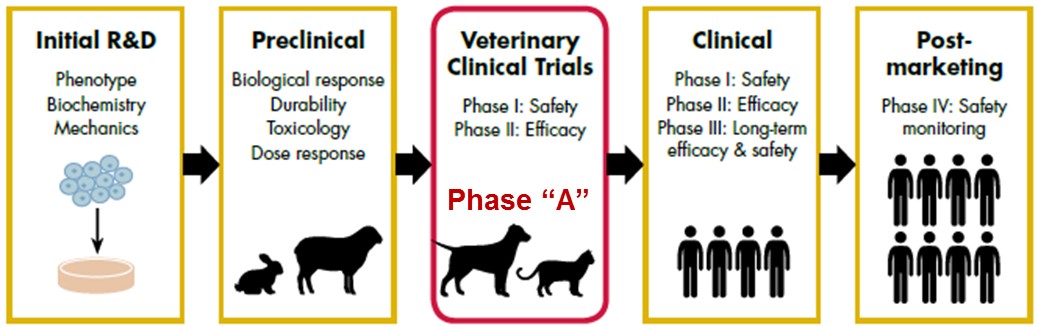
Here at the UC Davis School of Veterinary Medicine, we believe that this high rate of attrition can be overcome by inserting a new developmental phase into the drug development process. This “Phase A” stage would allow for the treatment of naturally occurring disease (comparable to a human disease) in client-owned animals when that disease exists. Conditions including oral inflammatory disease, inflammatory bowel disease, heart disease and spinal cord injury are just a few examples of diseases that occur in both animals and people. Given the diversity in the animal population (i.e. different breeds) we believe we can better predict the efficacy of drug therapies in humans while simultaneously developing new drug therapies for animals.